Molecular precision medicine in autoimmunity
Autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) arise from misguided immune responses against autoantigens, manifesting in a wide range of pathologic conditions with high socio-economic burden.
In the Heinz lab we combine classic biochemistry with contemporary “omics” approaches to chart the molecular mechanisms that characterize and drive these autoimmune diseases. The detailed understanding of underlying patho-mechanisms is key in identification of new drug targets as well as informative biomarkers enabling patient-specific precision medicine.
Focus I: Innate immune signalling pathways as drivers of autoimmunity.
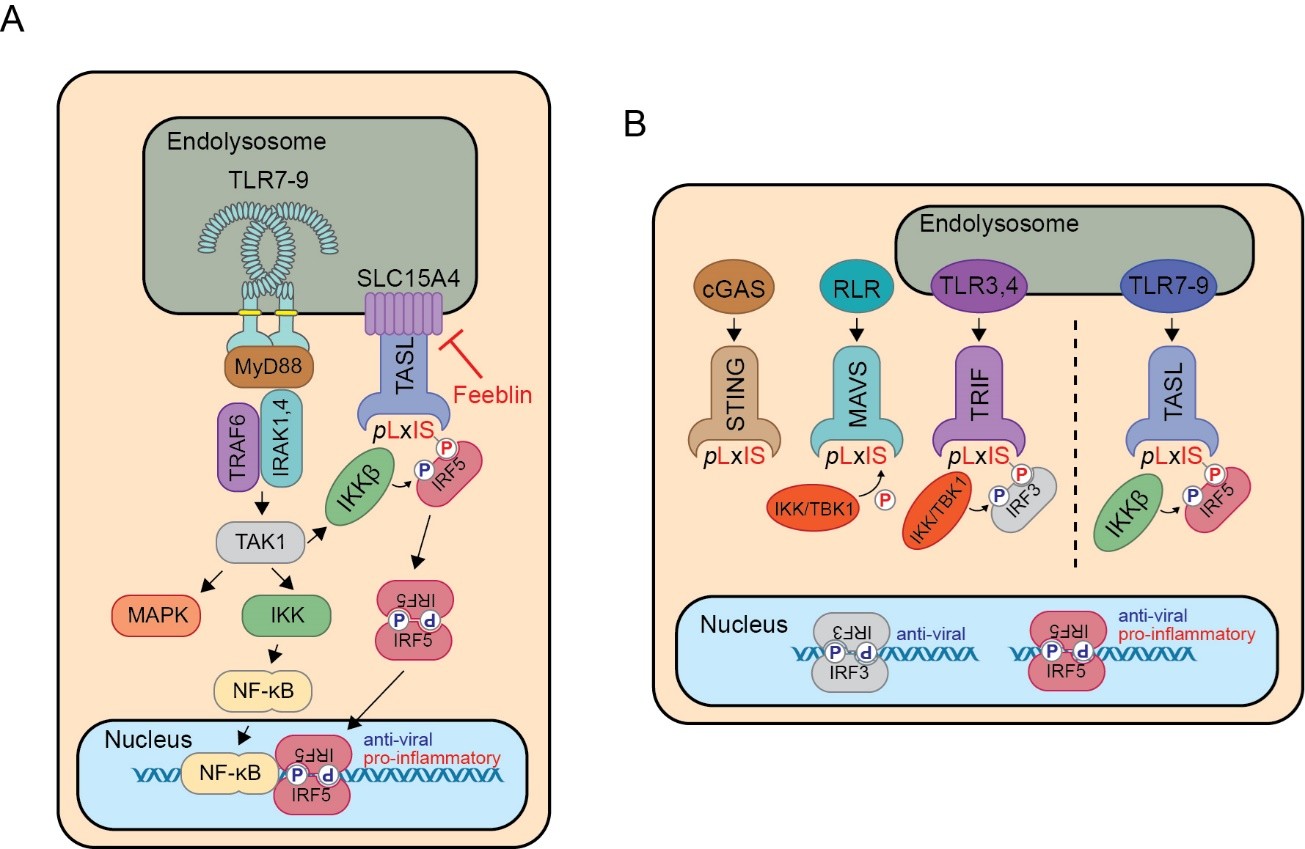
A major focus of the lab is on the SLC15A4/TASL adapter complex, which relays nucleic acid-sensing endosomal Toll-like receptor 7/8/9 signalling to IRF5 activation (Heinz et al., Nature, 2020; Figure 1). The strong genetic and functional link of the SLC15A4/TASL/IRF5 module to autoimmunity and recently demonstrated possibility of inhibition with first-in-class small molecules (Boeszoermenyi et al., Nat. Commun., 2023) makes it a prime target for further investigation in patients.
Figure 1 – (A) Schematic representation of the SLC15A4/TASL adapter module in endosomal TLR-induced IRF5 activation. (B) Comparison of the IRF3 adapters STING, MAVS and TRIF to SLC15A4/TASL-induced IRF5 activation.
Focus II: Phenotypic patient stratification by high-content microscopy.
High-content microscopy-based phenotyping of PBMCs from RA patients has been employed successfully for ex vivo drug testing and patient stratification purposes (Figure 2, Kartnig et al., eBioMedicine, 2025). This tool for functional precision medicine is currently further developed and tested, including in innovative co-culture models incorporating fibroblast-like synoviocytes (FLS).
Focus III: Structural immunology.
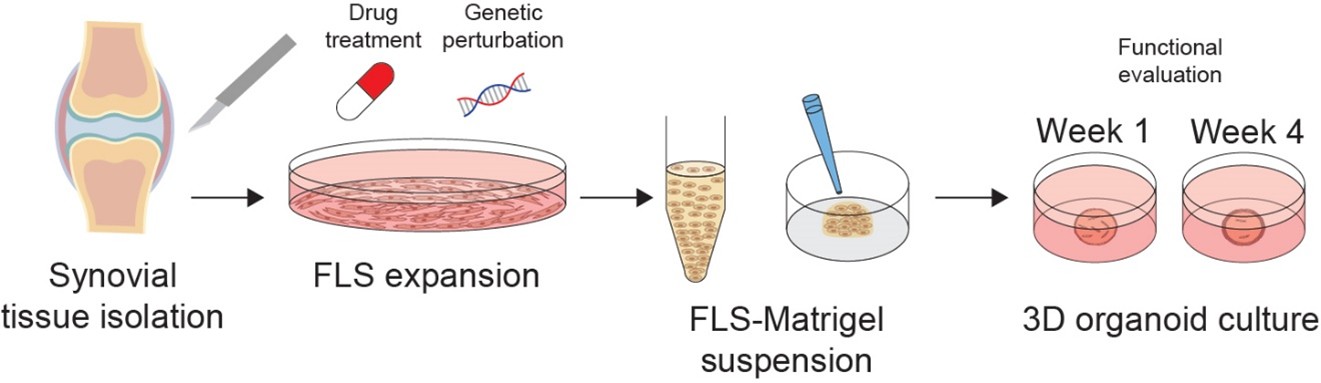
Fibroblast-like synoviocytes (FLS) are key drivers of rheumatoid arthritis (RA), yet therapeutics specifically targeting these cells are missing. In close collaboration with the experimental groups of at our division, we aim to employ functional approaches to elucidate mechanistic processes driving disease. Synovial biopsy-based innovative 2D and 3D synovial organ cultures are employed facilitating functional evaluation using CRISPR/Cas9-based approaches and drug testing (Figure 3).
Figure 3
Current members
As of May 2024.
Key publications
1. Kartnig, F. et al. Ex vivo imaging-based high content phenotyping of patients with rheumatoid arthritis. EBioMedicine 111, 105522 (2025). https://doi.org/10.1016/j.ebiom.2024.105522.
2. Boeszoermenyi, A. et al. A conformation-locking inhibitor of SLC15A4 with TASL proteostatic anti-inflammatory activity. Nat Commun 14, 6626 (2023). https://doi.org/10.1038/s41467-023-42070-3.
3. Mrak, D. et al. Heterologous vector versus homologous mRNA COVID-19 booster vaccination in non-seroconverted immunosuppressed patients: a randomized controlled trial. Nat Commun 13, 5362 (2022). https://doi.org/10.1038/s41467-022-33036-y.
4. Heinz, L. X. et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7–9. Nature 581, 316–322 (2020). https://doi.org/10.1038/s41586-020-2282-0.
5. Heinz, L. X. et al. The Lipid-Modifying Enzyme SMPDL3B Negatively Regulates Innate Immunity. Cell Rep 11, 1919–1928 (2015). https://doi.org/10.1016/j.celrep.2015.05.006.
6. Köberlin, M. S. et al. A Conserved Circular Network of Coregulated Lipids Modulates Innate Immune Responses. Cell 162, 170–183 (2015). https://doi.org/10.1016/j.cell.2015.05.051.