Rheumatic musculoskeletal disorders are the leading cause for pain and disability worldwide.
The most common inflammatory, erosive joint disorder is rheumatoid arthritis (RA). Chronic joint inflammation causes irreversible structural joint damage if the disease is untreated or not adequately responding to available therapeutic strategies.
Our scientific research focuses on the identification of novel pathomechanisms and targets contributing to inflammation-driven bone and cartilage damage. We aim to gain more insights into the local generation of synovial osteoclasts, the major cell type responsible for the occurrence of bone erosions. In particular, we study the association of a dysregulated immune system (pro-inflammatory cytokines, signaling pathways) and alterations of the skeletal system. Our research also addresses impaired osteoblast function under inflammatory conditions as well as the role of activated synovial fibroblasts on joint destruction.
To explore pathways of inflammation-mediated structural joint damage we run various in vivo models of RA investigating both gain-of function knockouts and therapeutic strategies. Major outcome measures include clinical signs, µCT bone analysis, histopathological analysis as well as in vitro cell function assays (osteoclasts, osteoblasts, fibroblasts, chondrocytes).
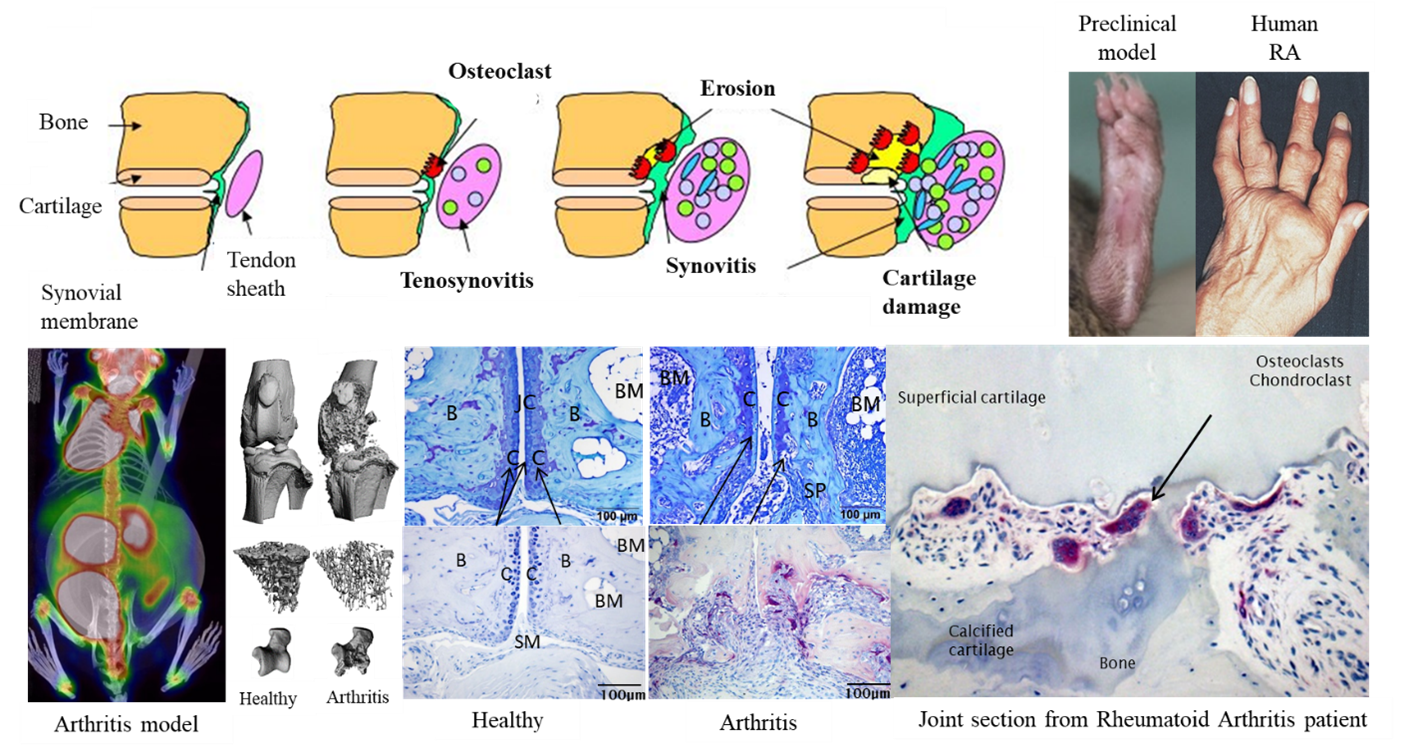
Images: Development of chronic inflammatory, erosive arthritis. Upper row: Schematic illustration of RA development. Clinical signs of swollen and deformed joints in a preclinical model and RA patient (right). Lower row (from left to right): [18F]PET/CT image of an arthritic mouse, µCT bone images of healthy and arthritic mouse knee, trabecular bone loss and talus bone damage; toluidine blue stained section indicating cartilage damage, tartrate-resistant acid phosphatase stained section indicating osteoclasts and subchondral bone erosion in an experimental mouse model. Right, human RA histological section indicating osteoclast and subchondral joint damage.
Current members
As of January 2024.

Publications Highlights
'SMASH' recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models.
This manuscript comprises international recommendations on standardized assessment of histopathological features in preclinical inflammatory, erosive arthritis models.
Multimodal [18 F]FDG PET/CT Is a Direct Readout for Inflammatory Bone Repair: A Longitudinal Study in TNFα Transgenic Mice.
In this study we used in vivo [18F]FDG PET/CT imaging to longitudinally monitor disease progression and treatment response indicating reversibility of joint inflammation and bone damage in an experimental arthritis mouse model.
Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis.
Using animal models allowed us to understand and identify first preclinical and very early histopathological events in inflammatory arthritis – a disease period which is not accessible from human patients.
Collaborations
- We are a member of the Ludwig Boltzmann Institute for Arthritis and Rehabilitation (LBIAR) exploring the role of glycobiology in experimental models of osteoarthritis, https://ar.lbg.ac.at/
- Our team is part of the bilateral Austrian-Hungarian Joint Research Project (Austrian FWF – Hungarian NKFIH, Projekt I5608) collaborating with the group of Tamas Nemeth, MD PhD (Semmelweis University, Budapest, Hungary) and Ulrike Steffen (Uniklinikum Erlangen, Germany) to investigate the role of dectin-2 in immune-complex mediated inflammation.
- We collaborate with several colleagues of the Medical University of Vienna (Stefan Tögel & Mario Rothbauer/Orthopedics; Erwin Wagner & Latifa Bakiri/Dermatology, Kazuhiko Matsuoka/Centre of Cancer Research; Bruno Podesser & Attila Kiss/Center of Biomedical Research and Translational Surgery) and University of Veterinary Medicine Vienna (Richard Moriggl/ The Functional Cancer Genomics laboratory).
External Funding
Our research is funded by the Austrian Science Fund (FWF), the Austrain Society of Rheumatology (ÖGR) as well as by industrial partners (MedTec, ElliLilly, and Abbvie).
Join us!
Join us! Master students of life science studies: contact silvia.hayer@meduniwien.ac.at with a CV & letter of intent detailing why you want to join the lab.